The US Food and Drug Administration has claimed that there were 55 reports of adverse events, including eye infections, permanent loss of vision, and even death from a bloodstream infection.
New Delhi,UPDATED: Feb 4, 2023 11:15 IST
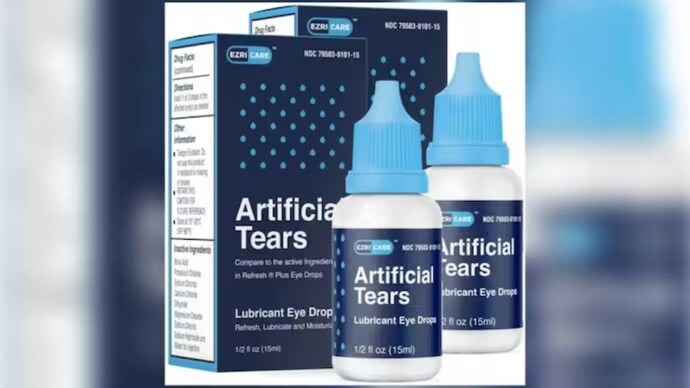
Artificial Tears Lubricant eye drops are used as a protectant against irritation or to relieve dryness of the eye.
By Milan Sharma: The Central Drugs Standard Control Organisation and Tamil Nadu’s Drug Controller conducted a late-night raid on Chennai-based Global Pharma Healthcare on Friday after US authorities flagged a possible contamination in a line of eye drops, sources told India Today.
Global Pharma Healthcare, located 40 km south of Chennai, recalled its artificial tears lubricant eye drops allegedly linked to vision loss and one death in the US.
The US Food and Drug Administration has claimed that there were 55 reports of adverse events, including eye infections, permanent loss of vision, and even death from a bloodstream infection.
READ | Indian drug firm recalls eye drop linked to vision loss in US
In a statement posted on its website, Global Pharma Healthcare said it is notifying the distributors of this product and is requesting that wholesalers, retailers and customers who have the recalled product should stop using it.
The US health regulator noted that “use of contaminated artificial tears can result in the risk of eye infections that could result in blindness.”
Artificial tears lubricant eye drops are used as a protectant against irritation or to relieve dryness of the eye. The US health authority claimed that the eye drops of Global Pharma Healthcare were contaminated with drug-resistant bacteria.
(With inputs from PTI)

